Ergonomics and Human Factor Research - Evaluation of Clinical Tool for Medication Preparation
Project Type
User-Centred Design, Human Factors, Ergonomics
Year
2025
Funding Sponsor
DAZL Innovation, Natural Sciences and Engineering Research Council of Canada (NSERC)
Introduction
It’s well known that nurses and personal support workers experience relatively high rates of work-related musculoskeletal disorders, and turnover among nurses and pharmacists remains a persistent challenge. These issues affect not only worker health but also organizational costs, with the turnover of a single registered nurse estimated at roughly $46,100. In many healthcare environments, opening sealed medication vials is a routine task that contributes to cumulative hand strain and increases the risk of work-related musculoskeletal disorders. During our research partnership with DAZL Innovations Inc.
To address these challenges, our research team collaborated with DAZL Innovations to evaluate The Lifty, a handheld device designed to reduce the physical effort required to open medical vials. Applying human factors principles and user-centred design, we conducted a two-phase study combining linear force testing, ergonomic and biomechanical modelling, and structured user-experience evaluation. This approach allowed us to quantify the physical demands of vial decapping, assess usability with real clinicians, and provide evidence-based recommendations without disrupting actual patient care environments.
The results showed that The Lifty significantly reduced the physical effort required to open vials, with force reductions of over 75 percent. Modelled muscle exertion remained well below ergonomic risk thresholds. Usability testing indicated that healthcare professionals found The Lifty highly intuitive, with a System Usability Scale score of 99.5 out of 100. Participants identified key design improvements and recognized the tool’s potential to address risks such as injury, product loss, and breaches of sterilization. Overall, findings support the effectiveness of The Lifty in reducing the physical demands required by healthcare workers to remove vial caps, which would be particularly beneficial to those most at risk for musculoskeletal injuries. A cost-benefit simulation projected a positive return on investment within 3.24 years of clinical use.
The following page will guide viewers through each stage of the research process, from defining the problem to validating The tool’s performance and understanding its impact on clinical workflows.
Materials & Methods - Linear Force Testing
A custom fixture was developed to secure bottles horizontally during force testing, with the bottles laid flat and oriented parallel to the floor to prevent any movement and minimize the during testing. Two testing conditions were assessed: one involving manual opening without assistance of The Lifty, and one with the assistance of The Lifty. When testing the force required to open the vial caps without the use of The Lifty, the flat press attachment was secured to the force gauge and the peak compression value was recorded. Force was applied through the force gauge to the outermost lip of the vial cap, on a line as parallel to the long axis of the bottle as possible, replicating the typical force vector produced by a user when opening a vial by hand. When testing the Lifty-assisted condition, bottles were secured into the custom fixture in the same orientation. The Lifty was applied to the vial cap such that The Lifty began in a position parallel to the gravity vector, with the opening of the tool oriented upwards and the handle in the downward direction. The hook attachment was then fixed through the hole in the handle, and force was applied as orthogonal to the long axis of The Lifty as possible throughout each trial.
Peak tension values were recorded and converted to linear force by dividing the torque by the length of the moment arm (the distance between the contact point of The Lifty on the vial cap and the point of contact with the force gauge). Under both conditions, force was applied until the foil seal was sufficiently broken, consistent with clinical practice. Force measurements were repeated for each bottle size until values were deemed stable (coefficient of variation < 20%). A Grubbs’s test (Grubbs, 1969) was used to detect and remove outliers prior to variance analysis
Materials & Methods - Physical Demand Modelling
HandPak software (HandPak, Potvin Biomechanics, Tecumseh, ON, Canada, 2025) was used to normalize the measured forces against established maximum capacities reported in the literature. The software generates task-specific strength estimates for hand and wrist actions, accounting for factors such as grasp type, force direction, duration, and the number of fingers involved.
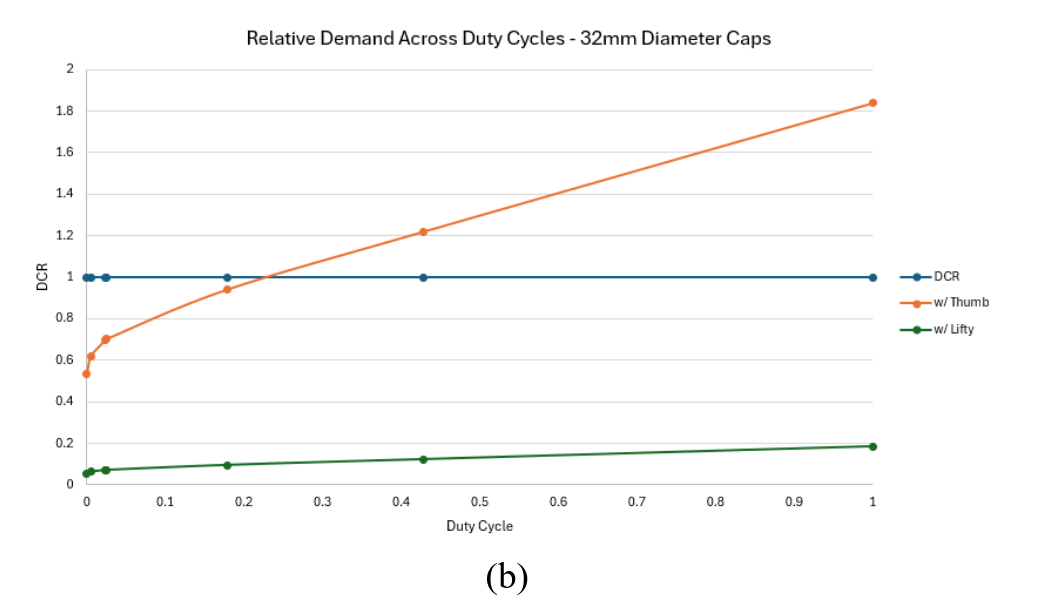
After the linear forces were collected under each condition they were averaged and normalized to the maximum human work published in the literature. The manual condition was modelled as a thumb press and the Lifty-assisted condition was modelled as a three-finger pull. Estimates of physical demand, expressed as percentage of maximum voluntary exertion (%MVE) were contrasted between conditions across a range of reported task frequencies (ranging in duty cycle 0.001 - 0.99). A ratio of demand:capacity (DCR) was calculated for each combination of condition and duty cycle. Capacity was modelled using the strength of a 25th percentile female.
Materials & Methods - Focus Group & Questionnaire
I had led a participant recruitment campaign over a three week period with inclusion criteria being an experienced healthcare worker or a student in training with prior experience removing vial caps. Participants were recruited via bulletin boards, emailing and online advertisements.
Focus group sessions were conducted with 1 to 3 participants per group in a conference room on Fanshawe College’s main campus, providing a controlled testing environment. Each session involved a moderated, task-based usability evaluation using a coached think-aloud protocol (Olmstead et al., 2010). Participants were guided through opening medication vials both with and without the tool while the facilitator encouraged them to verbalize their thoughts throughout the process. A second researcher observed the sessions and recorded behavioural responses and verbal feedback. After completing the tasks, participants filled out a structured questionnaire to capture further reflections and usability impressions. Each session lasted no more than 45 minutes.
The questionnaire included an adapted version of the system usability scale (SUS) (Brooke, 1996) and NASA Task Load Index (TLX) (Hart and Staveland, 1988).
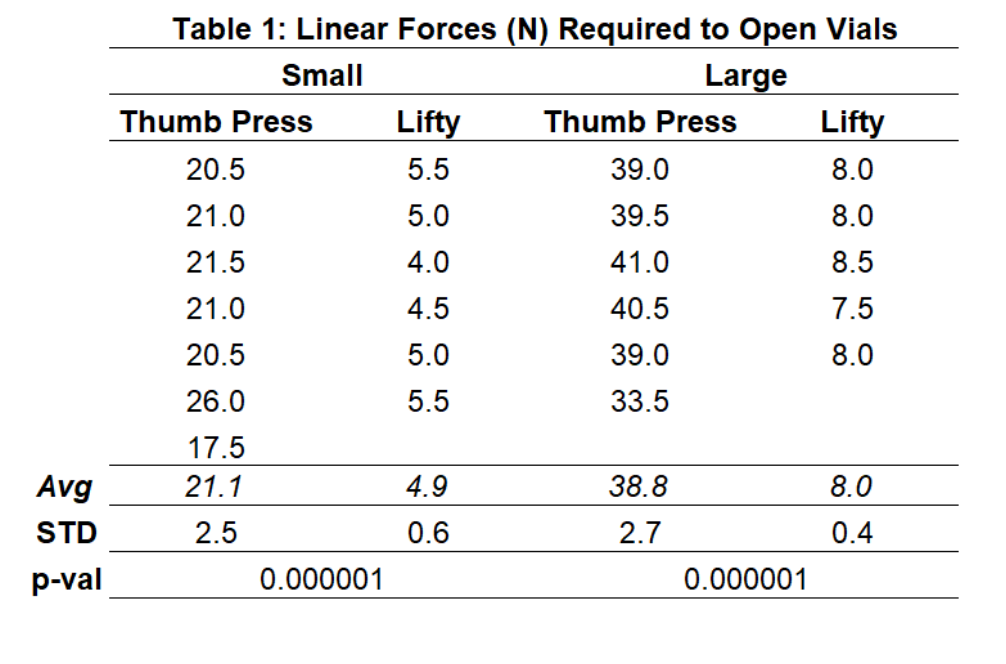
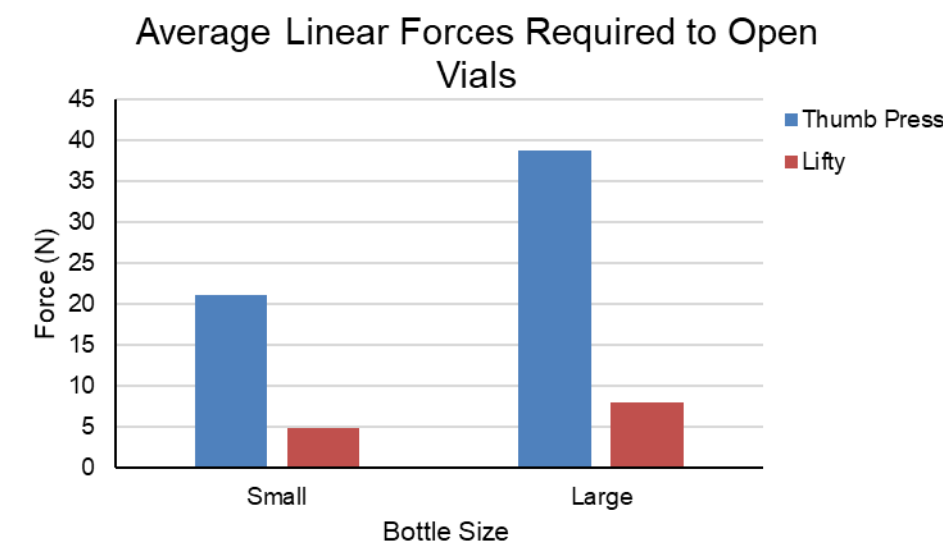
Results - Linear Force Testing
The linear force testing showed that The Lifty significant;y reduced (p<0.001) the linear force required to open medicine vials in both bottle sizes. For the large small bottles there was a 77% reduction of force and 79% reduction of force when using The Lifty.
Results - Physical Demands Modelling
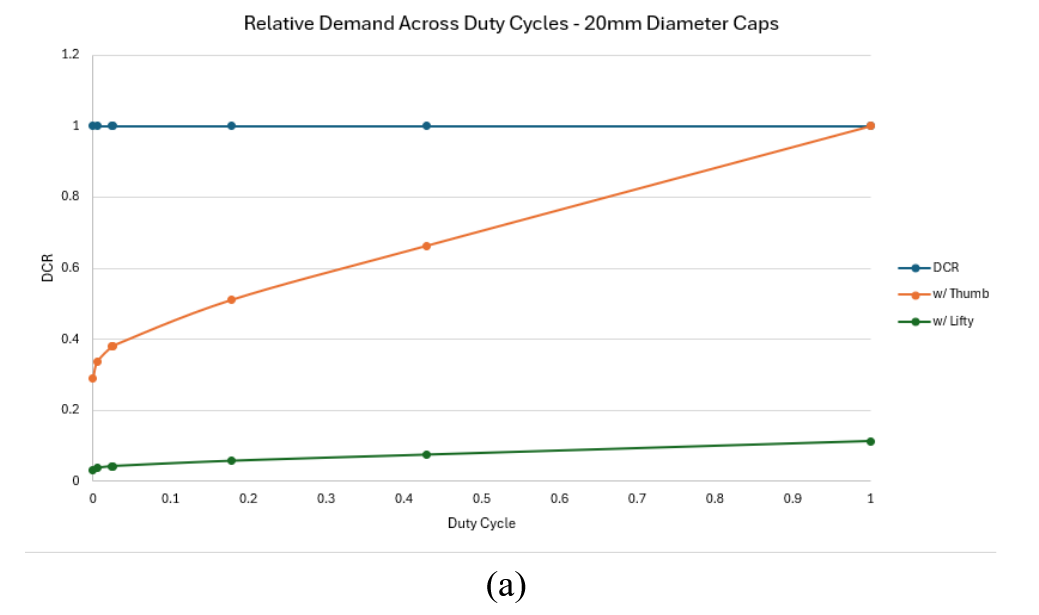
Physical demand modelling indicated that manual opening without The Lifty required a muscle contraction intensity of 26.5%MVE (maximum voluntary effort) and a demands capacity ratio (DCR) of 0.29. The This can be interpreted to mean that for very infrequent use (i.e., 10 de-cappings as day)the activity required to open the bottle uses approximately 30% (0.29) of an individuals work capacity for the entire day. As the duty cycle increases to around 100% (1.0) the duty cycle ratio draws near to the maximum acceptable intensity for a 25th percentile female indicating a increased risk of fatigue and/or discomfort.
For larger vial caps or infusion bottles physical demand modelling indicated manual opening without The Lifty required a muscle contraction intensity of approximately 49%MVE and a DCR of 0.53 ata low duty cycle (10 caps per shift). as the duty cycle approaches approximately 0,25 the level of exertion draws near to the maximum acceptable intensity allowable based on guidelines. Additionally the large bottle when opened without The Lifty Required about 40% more effort relative to the smaller vial caps on average
Using the Lifty for the small vial cap condition led to a reduction in muscle contraction intensity to 3%MVE and a DCR of approximately 0.03 for the small vial caps at a 100% duty cycle. For the large vial caps The Lifty reduced muscle contraction intensity to approximately 5% MVE and reduced the the DCR to under 0.1 for infrequent de-cappings. similar to The Lifty condition for the small vial caps at a 100% duty cycle the level of demand remains below estimated safety threshold and fatigue is not expected to occur even at very high repetition frequencies.
Results- User Experience Testing
The thematic analysis outlined who would use the device, how vial caps are currently removed, and the physical actions involved in both manual removal and using The Lifty. It also highlighted several challenges associated with the existing manual process and noted contextual constraints influencing how The Lifty is perceived. Insights pointed to differences between routine and urgent medical environments in terms of practicality and expectations.
From these findings, several design opportunities emerged. These included simplifying operation, improving portability, integrating with existing equipment, broadening functionality, enhancing visibility, and better aligning the device with both routine and high-pressure clinical settings.
The Lifty scored a 99.5 (range 97 - 100) on the revised SUS. Results from the TLX indicated no mental demand, physical demand, hard work, or frustration were introduced to the users while using The Lifty. All participants experienced perfect success in using the tool.
Conclusion
This project highlights a novel approach to integrating usability and ergonomics within healthcare environments. By gathering and incorporating feedback directly from employees, design ideas can be evaluated in a user-centered way. These proposed changes can then be tested through simulation or digital human modelling prior to implementation, helping to conserve resources and demonstrate their effectiveness. Identifying and addressing potential issues early not only reduces associated costs but also has a positive impact on key outcomes such as productivity, quality of care, and the likelihood of adverse events. This proactive approach supports safer, more efficient healthcare spaces that better serve both patients and staff.